Assessment of Drug Candidates’ Cardiac QT Liability
Before new drug candidates can be tested in humans, it is critical to assess whether they will provoke an irregular heart rhythm. The U.S. Food and Drug Administration requires preclinical testing on all drug candidates to ensure they don't cause QT interval prolongation on an electrocardiogram: an abnormality of the heart's electrical system that can lead to a potentially life-threatening arrhythmia and cause sudden death.

Yan's lab is available to pharmaceutical companies to test their drug candidates. For more information, email Gan-Xin Yan at [email protected] or call 484.476.2687.
Why choose Wedge Prep?
- Four different blinded tests have been conducted—by GSK, Johnson & Johnson, Novartis, and FDA scientists working with Yan's lab—that verified the wedge prep's superiority to other preclinical models in predicting the risk to humans from drug candidates, including ventricular action potentials, the isolated Langendorff perfused rabbit heart model, patch clamps for hERG current, and human-induced pluripotent stem cell-derived cardiomyocytes,
- Blinded tests have found the wedge prep resulted in zero false positives and false negatives—a record no other model has achieved.
- The wedge prep can also detect non-QT-related arrhythmias, such as those caused by Class Ic sodium channel blockade.
- The wedge prep is highly cost-effective because of its ability to distinguish a drug's effect on multiple ionic channels, including hERG current, INa, ICa,L and IKs.
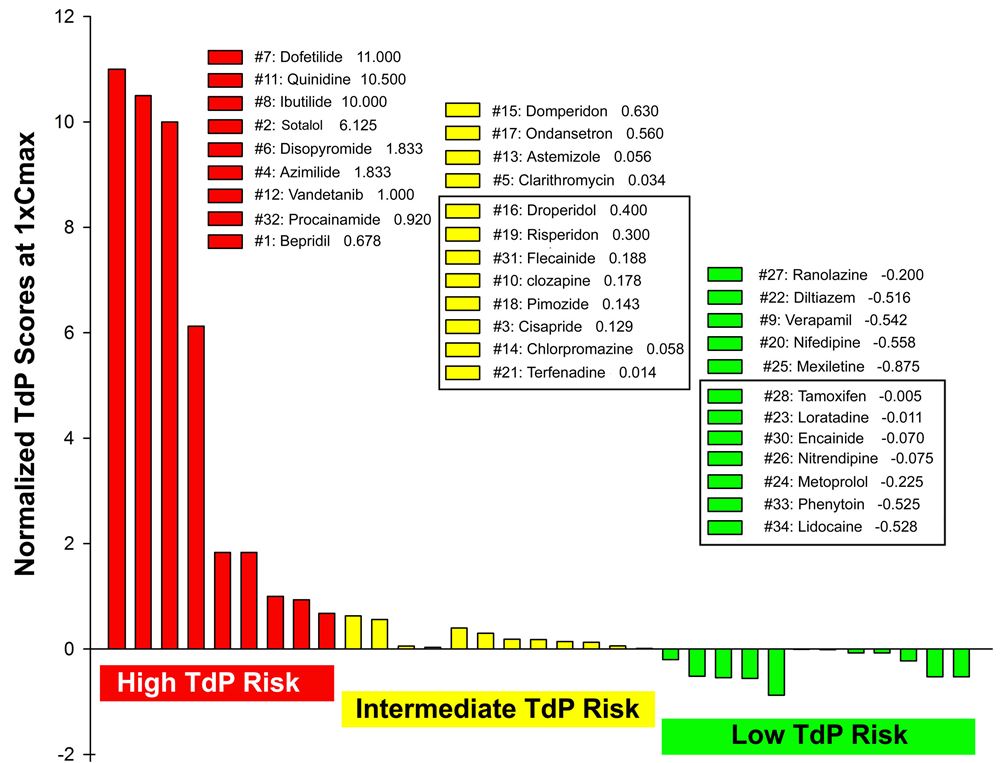
Normalized TdP scores based on wedge prep data at 1xCmax ;of 33 drugs, including 28 CiPA drugs
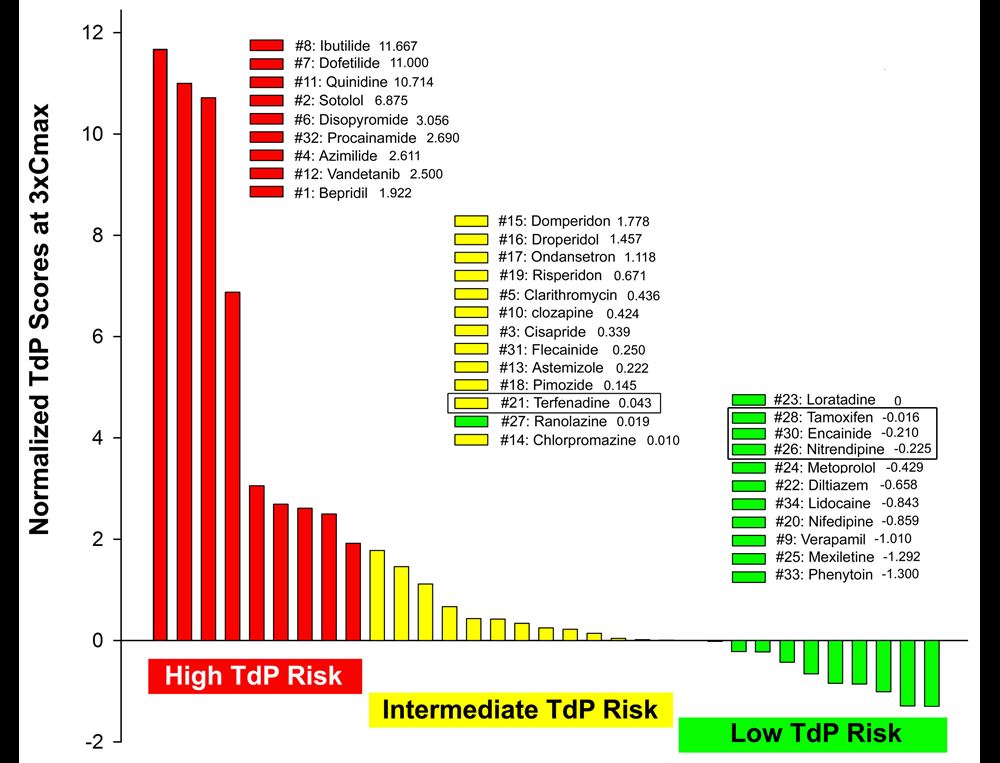
Normalized TdP scores based on wedge prep data at 3xCmax of 33 drugs including 28 CiPA drugs
References
Utility of Normalized TdP Score System in Drug Proarrhythmic Potential Assessment: A Blinded in vitro Study of CiPA Drugs. Liu T, Liu J, … Yan GX. Clin Pharmacol Ther. 2021 Jun;109(6):1606-1617.
Assessment of drug-induced proarrhythmia: The importance of study design in the rabbit left ventricular wedge model. Lu HR, Gallacher DJ, Yan GX. J Pharmacol Toxicol Methods. 2016 Sep-Oct;81:151-60.
Differentiating electrophysiological effects and cardiac safety of drugs based on the electrocardiogram: a blinded validation. Liu T, Traebert M, … Kowey PR, Yan GX. Heart Rhythm 2012 Oct;9(10):1706-15.
Preclinical assessment of drug-induced proarrhythmias: role of the arterially perfused rabbit left ventricular wedge preparation. Wang D, Patel C, … Yan GX. Pharmacol Ther. 2008 Aug;119(2):141-51.
Blinded validation of the isolated arterially perfused rabbit ventricular wedge in preclinical assessment of drug-induced proarrhythmias. Liu T, Brown BS, Wu Y, Antzelevitch C, Kowey PR, Yan GX. Heart Rhythm. 2006 Aug;3(8):948-56.
